The UK has become the first country in the world to approve the Pfizer/BioNTech coronavirus vaccine for widespread use.
British regulator, the MHRA, says the jab, which offers up to 95% protection against Covid-19 illness, is safe for rollout next week.
Immunisations could start within days for those who need it the most, such as elderly people in care homes.
The UK has already ordered 40m doses – enough to vaccinate 20m people.
Around 10m doses should be available soon, with the first 800,000 arriving in the UK in the coming days.
It is the fastest ever vaccine to go from concept to reality, taking only 10 months to follow the same developmental steps that normally span a decade.
Prime Minister Boris Johnson tweeted “It’s the protection of vaccines that will ultimately allow us to reclaim our lives and get the economy moving again.”
Health Secretary Matt Hancock told BBC Breakfast that people will be contacted by the NHS when it is their turn for the jab.
He said: “I’m confident now with the news today that from spring, from Easter onwards, things are going to be better and we’re going to have a summer next year that everybody can enjoy.”
NHS Chief Executive, Sir Simon Stevens, said the health service was preparing for “the largest-scale vaccination campaign in our country’s history”.
Around 50 hospitals are on standby and vaccination centres in venues such as conference centres are being set up now.
Although vaccination can start, people still need to remain vigilant and follow coronavirus rules to stop the spread, say experts.
That means sticking with the social distancing and face masks, and testing people who may have the virus and asking them to isolate.
England’s Chief Medical Officer Prof Chris Whitty said: “We can’t lower our guard yet.”
Prof Danny Altman, Professor of Immunology at Imperial College London, said news of the approval was “momentous”.
“We have the first emergency approval for use of a really effective vaccine. Truly heroic.”
Pfizer said it was a win for science.
What is the vaccine?
It is a new type called an mRNA vaccine that uses a tiny fragment of genetic code from the pandemic virus to teach the body how to fight Covid-19 and build immunity.
An mRNA vaccine has never been approved for use in humans before, although people have received them in clinical trials.
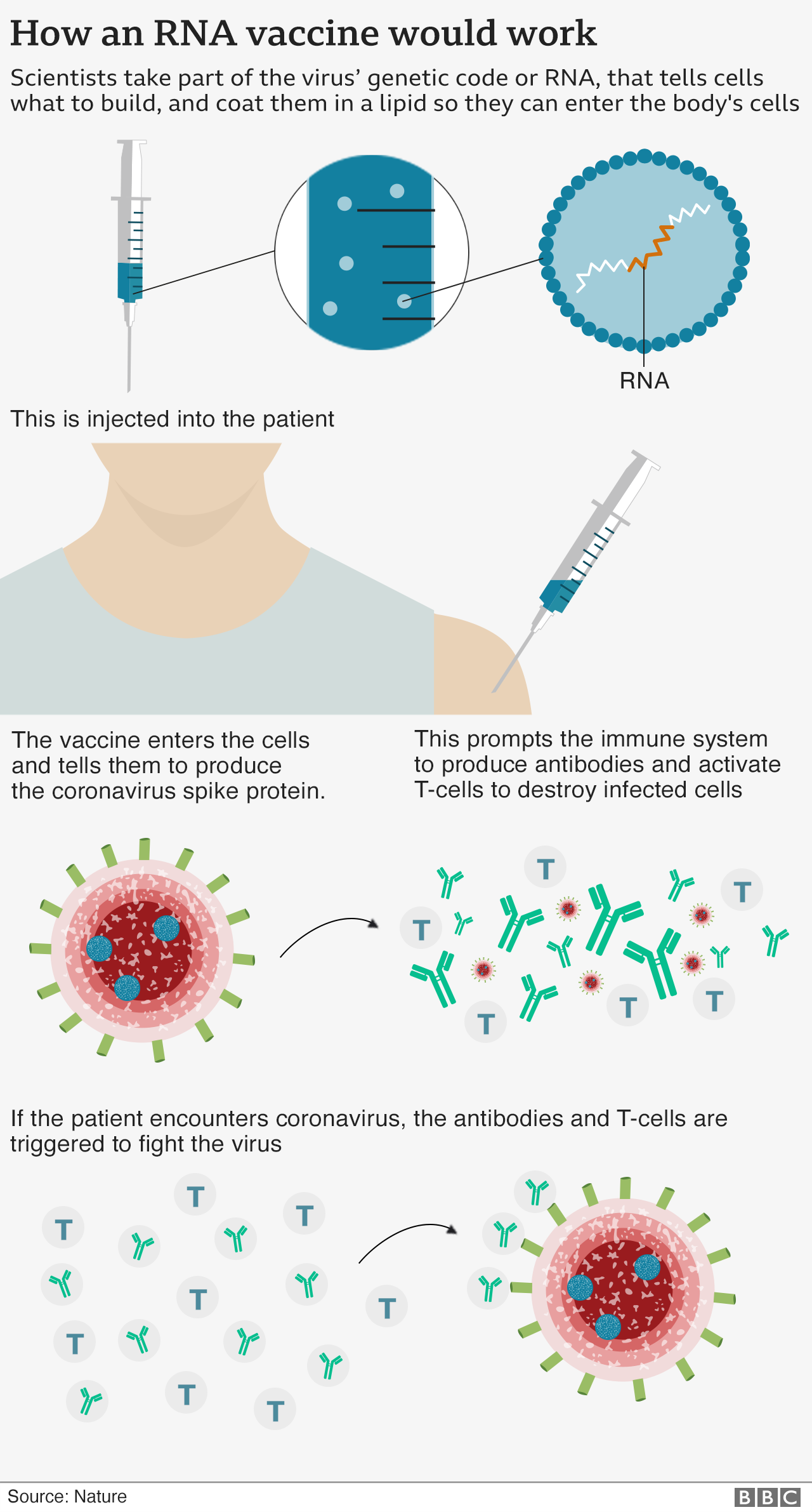
The vaccine, which is made in Belgium, must be stored at around -70C and will be transported in special boxes, packed in dry ice. Once delivered, it can be kept for up to five days in a fridge.
Who will get it and when?
Experts have drawn up a provisional priority list, targeting people at highest risk. Top are care home residents and staff, followed by people over 80 and other health and social care workers.
They will receive the first stocks of the vaccine – some as soon as next week. Mass immunisation of everyone over 50, as well as younger people with pre-existing health conditions, can happen as more stocks become available in 2021. It is given as two injections, 21 days apart, with the second dose being a booster.
What about other Covid vaccines?
There are some other promising vaccines that could also be approved soon.
One from Moderna uses the same mRNA approach as the Pfizer vaccine and offers similar protection. The UK has pre-ordered 7m doses that could be ready by the spring.
The UK has ordered 100m doses of a different type of Covid vaccine from Oxford University and AstraZeneca. That vaccine uses a harmless virus, altered to look a lot more like the virus that causes Covid-19.
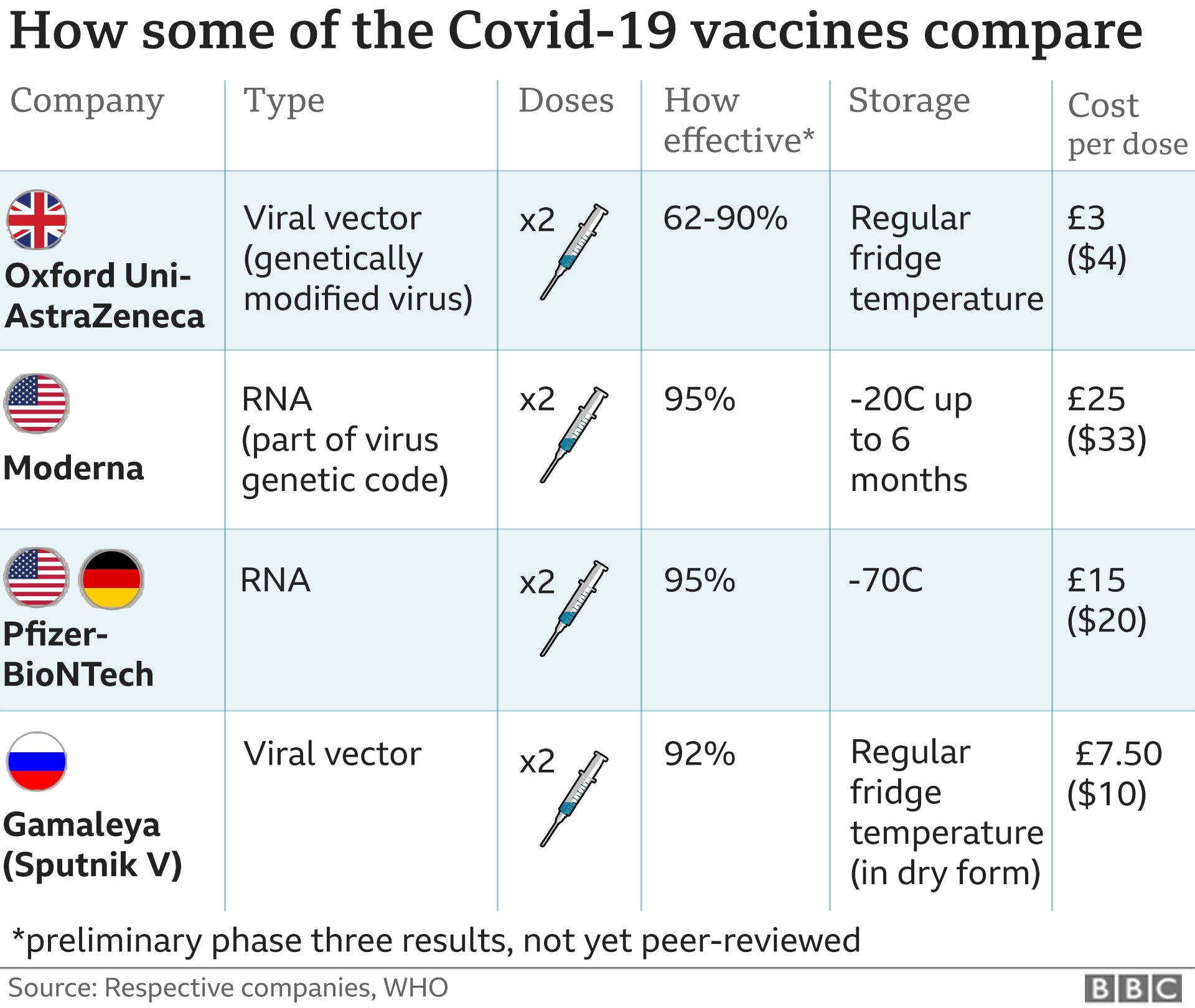
Russia has been using another vaccine, called Sputnik, and the Chinese military has approved another one made by CanSino Biologics. Both work in a similar way to the Oxford vaccine.